Abstract
Introduction: Despite numerous therapies approved for multiple myeloma (MM) in the past decade, the disease remains largely incurable. As a result, patients (pts) typically require successive lines of therapy, comprised of various combinations of drugs, to treat relapsed disease. As the treatment landscape evolves, continued real-world (RW) studies provide additional understanding of the treatment patterns of MM pts outside of clinical trials, particularly for those with unmet medical need and/or high-risk disease. In this retrospective, observational cohort study, we examined RW treatment patterns among MM pts overall and within patient subgroups classified by age, race/ethnicity, renal impairment (RI), cytogenetic risk, and 1q21 amplification, as well as patient outcomes.
Methods: This study used the nationwide Flatiron Health electronic health record-derived de-identified database of MM pts treated in the United States. During the study period, January 1, 2016 to April 30, 2021, pts who had ≥1 line of therapy or whose first-line treatment was initiated after the study start were included. Treatment class regimens (lines 1-4) were classified as: proteasome inhibitor (PI)-based, immunomodulatory drug (IMiD)-based, PI + IMiD-based, chemotherapy-based, antibody-based, or other treatments. We examined patient characteristics as well as treatment patterns overall and in patient subgroups. We also assessed real-world progression-free survival (rwPFS), defined as the time from start of line therapy to the date of progression or death, by treatment regimen received.
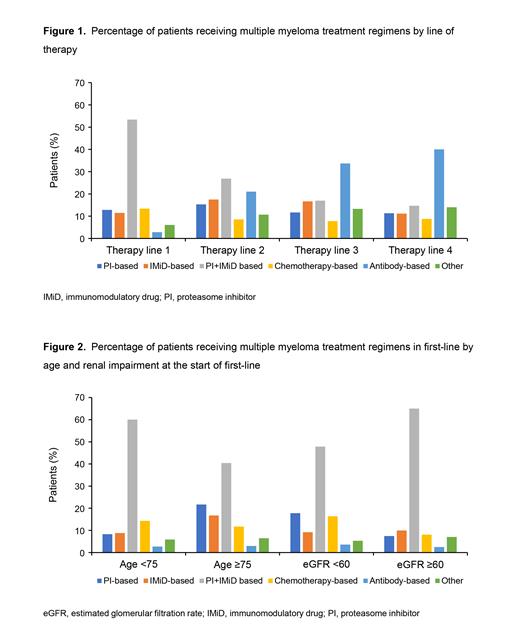
Results: At the time of data cutoff, 5465 pts received ≥1 line of therapy; 45.3% of pts were female, 57.4% were white, median age at the start of first-line therapy was 70 years (interquartile range 62-77), and 88.7% received care at community practices. A total of 14.6% had high-risk cytogenetic abnormalities, 21.0% had 1q21 amplification, 20.7% had International Staging System stage III at diagnosis and 33.2% had RI (eGFR <60 mL/min/1.73 m 2) at the start of first-line therapy. The most common first-line regimens were PI + IMiD-based (53.4%), whereas 12.8% received PI-based, 11.5% IMiD-based, 13.4% chemotherapy-based, 2.8% antibody-based, and 6.1% had other treatments (Figure 1). Although uncommon in first-line therapy, antibody-based treatments were more commonly used in later lines of therapy (second-line: 21.0%; third-line: 33.7%; fourth-line: 40.0%). In first-line, pts aged ≥75 years were less likely to receive PI + IMiD-based regimens than those aged <75 years (40.4% vs 60.1%) (Figure 2). Similarly, RI pts received PI + IMiD-based regimens less frequently in first-line than those without impairment (47.8% vs 65.0%). In first-line, 18.9% of pts had evidence of undergoing a transplant. Both older pts and those with RI were also less likely to receive a transplant as part of their first-line treatment (1.7% vs 27.7% and 13.9% vs 24.2% respectively). Treatment differences were less pronounced in later lines as well as by race/ethnicity, cytogenetic risk, and 1q21 amplification. In first-line therapy, rwPFS was longer for pts treated with PI + IMiD-based regimens (median [95% confidence interval], 29.5 months [27.3-31.9]). In later lines of therapy, pts treated with IMiD-based regimens had longer median rwPFS (second-line: 22.7 months [18.5-30.4]; third-line: 19.7 months [14.2-37.0]; fourth-line: 16.1 months [6.4-26.7]). Additional analyses examining newer treatment regimens will be presented.
Conclusions: PI + IMiD combination therapy was the most common first-line therapy. Although not commonly used in first-line therapy, antibody-based regimens are increasingly used in later lines of therapy. Older pts as well as pts with RI were less likely to receive PI + IMiD regimens or transplant in first-line therapy. Pts receiving PI + IMiD regimens in first-line therapy had longer rwPFS. Pts receiving IMiD-based regimens had longer rwPFS in later lines. Analyses of newer treatment regimens to be presented within this dataset will provide additional insight into changes in RW treatment patterns in the era of novel agents.
Richter: BMS, Karyopharm, Antengene: Membership on an entity's Board of Directors or advisory committees; Adaptive biotechnologies: Speakers Bureau; Janssen, Celgene: Membership on an entity's Board of Directors or advisory committees, Speakers Bureau; Tisch Cancer Institute: Icahn School of Medicine at Mount Sinai: Current Employment. Singh: Sanofi: Current Employment. Rice: Sanofi: Current Employment, Current holder of individual stocks in a privately-held company.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal